 |
| Figure-01: N-Nitrosamine impurities: An overview |
Nitrosamine impurities, More correctly N-nitrosamine
impurities, Now a days very hot and burning issue in the pharmaceutical
industry and a topic for curious discussion among the pharmaceutical
professionals. So for the understanding on what actually nitrosamine impurities
are and how it is having critical and adverse impact on human health, we have
taken a brief review on this topic. I hope you all will like it.
In this article we will have an insight view on nitrosamine impurities for; its chemistry, behavior, acceptance etc. We will have answers of basic questions like what it is exactly, what is origin /source and allowable daily intake for nitrosamines.
Basically Nitrosamines (Chemical Compound) are classified as member of carcinogenic and mutagenic impurities by International Agency for Research on Cancer (IARC). These chemical compounds are containing nitroso functional group, usually called as carcinogenic agent.
 |
| Figure-02: Nitroso group |
When nitrosamines were identified in
Pharmaceutical drug substance or drug products?
Before June-2018, presence of nitrosamine in the drug product and drug substance was not known. In the mid of year 2018, presence of these unexpected and undesired impurities were acknowledged and highlighted by EU authorities and FDA in drug product containing valsartan Active Pharmaceutical ingredients (API). FDA was informed of the presence of an impurity, identified as N-nitrosodimethylamine (NDMA), from one valsartan API producer.
These impurities are identified in various “-Sartan” based drug products around the world. Sartan-containing medicinal products (known as ARB) are important treatment options of serious or potentially serious conditions such as hypertension or certain heart or kidney diseases. More recently, nitrosamine impurities have been reported in other drug products as well containing drug substance like renetidine, nizatidine and metformin etc.
Various Pharmaceutical regulatory authorities around the world, taken regulatory actions like imposing recall on drug products containing impacted drug substance and stopping the use of active substances from certain manufacturers, were taken to reduce human risk.
A subsequent review and investigation was performed by the medicine authorities, which led to new requirements for pharmaceutical manufacture. These steps are taken for the awareness and educating the healthcare professionals, manufacturers and public as well about the adverse effects on nitrosamine (Carcinogenic). We will further know, what are the regulatory expectations, guidance of various medicine authorities on it?
What is Nitrosamines and it’s impurities?
Nitrosamines are the common chemical classified as a probable human carcinogen (a substance that could cause cancer) when ingested at higher level, very small exposure of these impurities can lead the cancer. ICH M7 (R1) classifies Nitrosamine impurities as Class 1, which is known to be mutagenic and carcinogenic.
These Nitrosamine impurities impact the genetic material by means of mutations through chromosomal breaks, rearrangements, covalent binding or insertion into the DNA during replication. These changes in the genetic materials caused by the exposure to very low levels of Nitrosamine impurities can lead to cancer.
The various regulatory authorities have published the press release or notice in regards to control of these impurities with interim control limit. Below is the list of possible nitrosamine impurities.
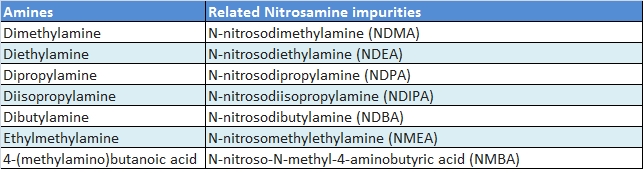 |
| Table-01: List of Amines and related Nitrosamine impurities |
The methodology guiding decision making on immediate market actions in case of nitrosamine contaminations has so far followed the ICH M7(R1) approach.
What is the source for N-nitrosamines impurities?
Nitrosamine impurities are the possible byproducts produced in trace amounts during the manufacturing processes of Drug substance, however based on the study various source could be the contributing factor for the introduction of these carcinogenic impurities in the drug substance or drug products.
The exact sources of these trace impurities have not been investigated, source could be the Manufacturing process of drug substance, direct introduction through the input materials, Degradation or cross-contamination. So in this view for the identification of the source of this impurity, it is important to perform a detailed investigation and risk assessment for the same.
|
In February 2020, Active Pharmaceutical Ingredients Committee (APIC) has published and guidance on risk assessment for presence of N-nitrosamines in drug substance along with the template for it. In June 2020, European medicine agency published an assessment report on N-nitrosamines impurities. In September 2020, FDA released Guidance for industry on control of nitrosamine impurities in human drugs. |
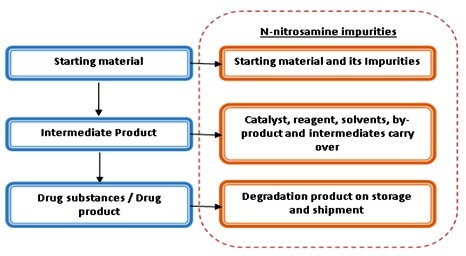 |
| Figure-3: Possible sources of N-nitrosamine impurities |
Manufacturing process could be the possible source of nitrosamine impurities, Nitrosamines are chemically simple molecules and can be formed during pharmaceutical drug substance manufacturing steps whenever there is a presence of a secondary (or tertiary) amines and nitrites, usually in acidic conditions.
Primary, secondary, tertiary amines or quaternary ammonium salts along with nitrosating agents such as Sodium nitrite are considered to be precursors for the generation of Nitrosamines impurities.
The extent of Nitrosamine impurity formation depends mainly on the type of reagent, their structure and the concentration of the nitrosating agent. Secondary amines are considered to be more reactive.
 |
| Figure-4: formation of N-nitrosamine impurities |
If we explore the possibility for the presence of these impurity that could be due to the direct introduction in drug substance or drug products.
Recovered solvents, catalysts used in the process may contribute in the process of impurity formation during the manufacturing of Drug substance. High risk and possibility via recovered solvent is supportive with the fact that during recovery process Nitric acid or sodium nitrite is being used to kill the residual azide. So, the presence of –nitroso group here is enough to cause formation of these impurities.
Keys starting material, raw material and excipients could be the source of nitrosamine impurity in drug substance or drug products, if contaminated material is supplied by the vendor. Hence, this point could not be overlooked.
Cross contamination between manufacturing process and products on the same production block / line may be the cause of contamination of N-nitrosamine. If in the process nitrosating agent is present may cause formation of by product as nitrosamine impurity, with the trace amount of nitrite present in water used in the process.
Degradation or decomposition phenomena in the chemical compound / material may lead the cause for the presence of nitrosamine impurity in the drug substance.
For instance, trace amount of this undesired impurity may be generated due to decomposition of solvent or other materials used for the synthesis of drug substance any carry forward to the drug substance.
via Packaging material, Possibility of impurity introduction in the drug substance or product cannot be ruled out due to the certain packaging materials containing nitrocellulose and may react with the precursors for the impurities i.e. amines present in the printing inks [dimethylamine (DMA) and diethylamine (DEA)].
In September 2019, a new root-cause for contamination of medicinal products with NDMA/NDEA was identified and reported to the authorities. NDMA/NDEA appear to have been formed during printing of the lidding foils and that formation of N-nitrosamines was caused by reaction of nitrocellulose in the lidding foil with amine containing printing ink [dimethylamine (DMA) and diethylamine (DEA)] and transferred to the finished product during heat-sealing blistering process via vaporization and condensation on the finished product.
Final Words, I Hope this article helped you to get an overview on the burning issue in current scenario further review is ongoing in the view of various pharmaceutical regulatory requirement and consideration on this topic and well get back with new stuff….
Reference Doc.: Article, FDA, EU, ICH, WHO, APIC, EMA, Guidance
Learn more……..
- What are Sartans (A family of Medicine)
- Process Validation study- Types of validation and advantage
- What is Validation study- Basic overview
- Product Lifecycle management- An overview
- What is Pharmaceutical Management system for Pharmaceutical Industries
- Pharmaceutical Quality Management system- An Overview
