Regulatory Affairs is a
profession, known as a bridge / a channel of communication, between the
Regulatory Agency and the organization seeking approval for new pharmaceutical
products.
It plays an important role in Product Life Cycle management stages from “Drug development” to “Commercialization”. For the
getting approval of new Products in various countries all over the world, there
is an approval procedure; having some set of procedures governed by the
respective country regulatory agencies.
New pharmaceutical products
registration is required with the complete details of Product and manufacturer
in the form of a dossier, The process of registration of Pharmaceutical Drug Substance / Drug Product in US market called as “New Drug Application (NDA)” and for
European countries it is called as “Marketing Authorization Application (MAA)”.
 |
| Regulatory Dossier in Pharmaceutical Industry |
❓ What is Regulatory Dossier?
When we find the dossier meaning in pharma, The
term “Dossier” is very important in the pharmaceutical industry it is an apex document
in the pharma called as Dossier. It is a collection of data and information of
pharmaceutical Drug, required to get the registration of drugs / to get
marketing approval from various regulatory agencies
around the world.
This process is called as “Marketing approval or
Registration”, “Marketing Authorization or “Product Licensing”. Dossier has
complete history of the Product, subjected for the Marketing Approval and
dossier submission is done after the successful completion of the phase III clinical
trial.
In simple words “Dossier” may be called as “Registration
Dossier”, which contains all technical details regarding the targeted
pharmaceutical product, seeking the approval for marketing in the respective
country/region.
Technical details expected in the dossier includes
administrative information about the manufacturer, Quality details (CMC),
non-clinical study details and clinical study details.
Dossier submission done
in US market for any new product called as “New Drug Application (NDA)”
and for European countries it is called as “Marketing Authorization
Application”. For other countries it is called as simply “Registration
Dossier;”
“The collection of informative documents / information used as a supporting document for “Regulatory Submission Application”, this grouped / collective documents called as Regulatory Dossier.
❓ What
is the purpose of regulatory Dossier Preparation?
Regulatory Dossiers preparation is being performed for the New Drug
applications, and it is required to get the Marketing Authorization i.e.
License to market the product in particular market.
Simply, regulatory dossier is submitted by the applicant to the
regulatory agency in order to obtain approval for marketing the product. These New Drug Applications are termed differently in different
regions/countries. Like;
✅ In Japan it is called as JNDA (Japan New Drug Application),
✅ In Canada as NDS (New Drug Submission),
✅ In USA as NDA (New Drug Submission)
✅ In
European Countries as MAA i.e. Marketing authorization Application”.
Additionally, it can be prepared for the update on existing submitted
dossier, where in update is required or proposed from manufacturer regarding
update in any information submitted previously to the respective regulatory
agency.
❓ What
type of information A Regulatory Dossier has?
Chemistry (CMC), Formulation,
Manufacture, Toxicology, Pharmacology, Pharmacokinetics, Clinical studies
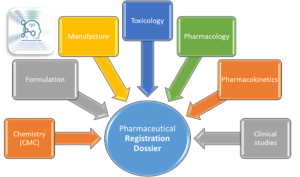 |
| Contents of Regulatory Dossier |
❓ What
is format and content for Regulatory Dossier?
The process of dossier submission may vary from one country to another
country / region. As the globalization of pharmaceutical industry occurs, it
has created the need to harmonization for the development of new
pharmaceuticals, as well as the regulatory requirements of various countries.
Thus, a
common format of submission has been developed through ICH process, the CTD’s
guidance have been developed for Japan, European Union, and United States.
Almost Most of the countries have adopted the CTD format.
However, when we deep dive in the subject we found that; basically,
there are two types of Regulatory dossier formats are available for the dossier
preparation i.e. ICH
CTD and ACTD (Asean Common Technical Dossier).
The ICH CTD formats
generally used by the ICH countries and developing countries and ACTD format is
being used by the group of Asian countries having ten members as Brunei,
Cambodia, Indonesia, Lao, Malaysia, Myanmar, Philippines, Singapore, Thailand,
Viet Nam.
❓ What is CTD (Common technical Dossier)?
This is a well-organized table of Content for Technical
Information on any “Pharmaceutical product” seeking the approval for
the marketing in the specific region / market. As we discussed above the
organization of ICH CTD is done in 5 modules to gather the information as one.
🔑 Module-1: Regional and Administrative Information
🔑 Module-2: CTD Overview and Summaries
🔑 Module-3: Quality
🔑 Module-4: Non-clinical study details
🔑 Module-5: Clinical study details
There is no difference with respect to the required information on new
pharmaceutical drug products, though arrangement of information may be
different, like;🔅 ICH CTD has 5 module within it for the arrangement of information.
🔅 ACTD has 4 Part for the collection of information
For the ICH CTD dossier preparation guidance is published by ICH as ICH-M4,
The ICH CTD is organized into five modules. Module 1 is region specific and
Modules 2, 3, 4 and 5 are intended to be common for all regions.
In July 2003,
the CTD became the mandatory format for new drug applications in the EU and
Japan, and the strongly recommended format of choice for NDAs submitted to FDA,
United States. This ASEAN
Common Technical Dossier (ACTD) is a guideline of the
agreed upon common format for the preparation of a well-structured Common
Technical Dossier (CTD) application that will be submitted to ASEAN regulatory
authorities for the registration of pharmaceuticals and biologics for human
use.
Learn More 📗📘📙:
What
is Marketing Authorization in Pharmaceutical Industry?
What
is the difference between clinical trial & clinical research?
The
Evolution of Drug Substance Development
Drug
Substance in Pharmaceuticals!! Unveiling Its Vital Role

