In
General terms Impurity is referred as something, which is not pure. Some
substance / component that may affect the SISPQ
(Safety, Identity, Strength, Purity and Quality) of a
drug substance/drug products (Ref.USP). Therefore, studies of impurities are one of the most important works
in the development of APIs and drug products.
We
will understand the below details under this article;
· Definition
of Impurities
· Classification
of Impurities
· Regulatory Requirements
· Risk assessments & Control strategies
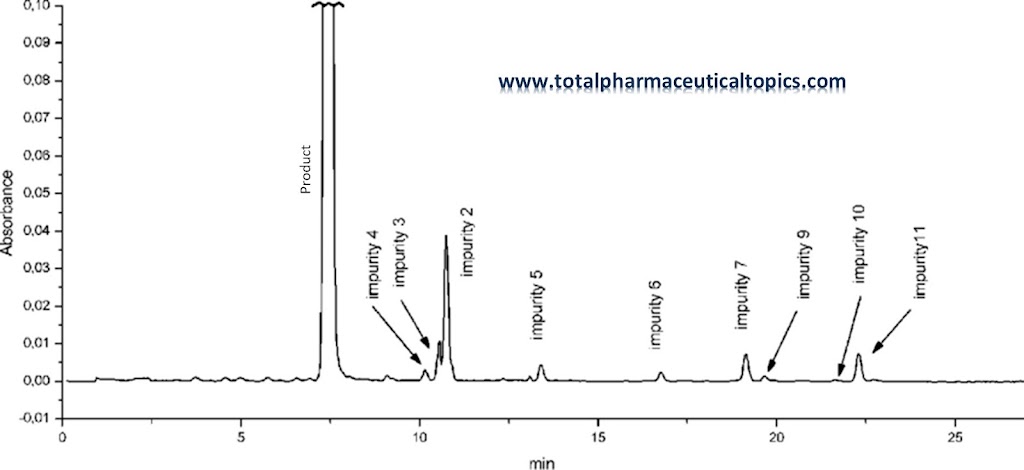
 |
| Impurities in Drug Substance and Control Strategies |
Definition of Impurities
In
the Pharmaceutical manufacturing, impurities can be defined as “component that is not the desired chemical
entity” expected as Drug substance. These can be sourced by Starting
material, raw material, Intermediate or during manufacturing operation of drug
substance resulting by-product.
As per ICH (International council for
Harmonization of Technical Requirements):
ICH
has published a specific guidance document on “Impurities” as ICHQ3, wherein it is defined that “Any component of the new drug substance
that is not the chemical entity defined as the new drug substance”.
As per WHO (World Health Organization):
“Any
component present in the drug substance or drug product that is not the desired
product, a product-related substance, or excipient including buffer components.
An impurity may be either process- or product-related.” (ref.: WHOTechnical Report Series No. 987)
Classification of Impurities
Based
on the Nature and Origin of the impurities, it can be classified under
following categories;
· Organic
Impurities
· In-organic impurities
· Residual Solvents
As
per ICH Q3 source of the organic
impurities can be the material used for the Drug substance manufacturing
like Starting material, reagents, ligands, catalysts, intermediates, and can be a degradation product of the desired
chemical entity / drug substance.
As
we are discussing the Organic impurity, two terms i.e. PRIs (Process related impurities) & DRIs (Degradation related impurities) are very important, this
classification helps us to develop the control strategy in terms of Analytical
method establishment and process improvement.
On
other hand, Source of In-organic
impurities can be Heavy metals or other residual metals Inorganic salts,
other materials (e.g., filter aids, charcoal), reagents, ligands and catalysts.
Solvents
are the liquid media used for the preparation of solution / suspension during
drug substance manufacturing process, these can be Organic or In-organic.
These are the general classification of
impurities based on the chemistry aspect. Can be understand better with below;
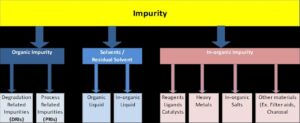 |
| Classification of Impurities |
Further in current scenario, impurities are also classified in other categories (based on their structure-activity relationship) and being evaluated, monitored and controlled at various steps/stages of drug substance synthesis / manufacturing. Some of these are;
· Elemental
impurities
· Nitrosamine impurities
· Genotoxic
impurities
· Mutagenic
impurities
· Azide
impurities
· Carcinogenic
impurities etc.
Regulatory Requirements
Various
guidance documents are made available by Regulatoryagencies worldwide, for evaluation and control of impurities in drug
substance as mentioned above. To meet the regulatory requirements on this it is
very important to classify the impurity along with the source identification.
Basic
requirement of regulators to perform Impurity determination with the sound
scientific / technical rational, knowledge and study. Guidedby FDA as well for the ANDA sponsors. It is expected by the regulatory
agencies that actual and potential impurities most likely to arise during the
synthesis, purification, and storage of the new drug substance (leads DRIs) is
studies and control strategy is planned.
Sound
scientific appraisal is expected, in terms of study of the chemical reactions
and storage conditions. Laboratory studies can be the pivot of that, to detect
impurities in the new drug substance based on knowledge of the chemical
reactions and conditions involved within.
 |
| Regulatory requirements |
Detection
and Quantification of impurities need to be established with the appropriate
analytical methods developed based on the validation studies i.e. Analytical
Method development and validation. (ref. ICHQ2 and ICHQ14).
After Detection, Quantification of impurities and development of
respective suitable analytical method; Listing of Impurities in Specifications
are also expected. That can be established with the details of the analytical
results obtained during the Stability studies, chemical development studies,
and routine batch analysis.
At
last, these identified /un-identified, specified/un-specified impurities should
be qualified with the rationale for establishing impurity acceptance criteria
that includes safety considerations as applicable.
Risk assessments & Control strategies
Conducting
a thorough risk assessment is crucial to identify potential impurities and
their associated risks early in the drug development process. This helps in
implementing appropriate control strategies.
Control
strategies for impurities involve a combination of techniques including
selecting high-quality raw materials, optimizing manufacturing processes,
employing appropriate analytical methods for detection and quantification, and
implementing effective purification techniques.
As
said by the requirements of ICH Q3A(R2), all types of impurities present in API
at a level greater than (>) the identification threshold must conduct
studies to characterize their structures, no matter they are shown in any batch
manufactured by the proposed commercial process or any degradation product
observed in stability studies under recommended storage conditions.
In Brief
In
the pharmaceutical manufacturing impurities and management of impurities must
be exercised and executed in strict compliance with the regulatory requirements
due to their quality and safety concerns. Here we tried to summarize the basic
understanding about the Impurities in pharmaceutical drug substance
manufacturing and control strategy.
Keep
Learning…..
New
Chemical Entities (NCEs) in Pharmaceutical Drug Development
Clinical
Trials: A Key to Pharmaceutical Development / Advancement
What
is Marketing Authorization in Pharmaceutical Industry?
Research
and Development: Definition
What
is the difference between clinical trial & clinical research?
Drug
Substance in Pharmaceuticals!! Unveiling Its Vital Role