In the ever-evolving landscape of the
pharmaceutical industry, ensuring the quality and reliability of processes is
paramount. One crucial aspect that stands at the forefront is the process
validation—a meticulous journey that guarantees the efficiency and safety of
pharmaceutical manufacturing.
 |
| 10 Key Steps for Successful Process Validation in Pharma |
In this article, we will unravel the intricacies
of process validation in pharma,
exploring the 10 key steps that pave the way for success. From understanding critical process parameters to
navigating FDA regulations, let’s embark on this journey together.
Step 1: Setting the Foundation –
Step 2: Navigating the Regulatory Landscape –
Step 3: Identifying Crucial Factors –
Step 4: Crafting the Blueprint –
Step 5: Unveiling the Lifecycle –
Step 6: Documenting the Journey –
Step 7: Ensuring Compliance –
Step 8: Mastering Precision –
Step 9: Integrating Statistical Wisdom –
Step 10: Achieving Continuous Improvement –
—————————————————————————————————————————————
Step
1: Setting the Foundation –
Understanding Pharmaceutical Process Validation Before diving into the specifics,
let’s establish a solid foundation by comprehending the essence of pharmaceutical process validation. This
step involves recognizing the significance of validation in maintaining product
quality and patient safety.
 |
| Fig. 01: Setting the Foundation Successful Process Validation in Pharma |
At its core, process validation is the systematic assessment and documentation
of a manufacturing process to ensure that it consistently produces a product of
predetermined quality. It’s not merely about meeting regulatory standards but
also about optimizing processes for efficiency, reducing risks, and ensuring
the reliability of every pharmaceutical product that reaches the market.
At its core, process validation is the systematic assessment and documentation
of a manufacturing process to ensure that it consistently produces a product of
predetermined quality. It’s not merely about meeting regulatory standards but
also about optimizing processes for efficiency, reducing risks, and ensuring
the reliability of every pharmaceutical product that reaches the market.
For
Understanding Basics on Process validation Learn More…….;
@ What is Validation? Basic overview
@What is Process Validation? What are the Types of validation?
—————————————————————————————————————————————
Step
2: Navigating the Regulatory Landscape –
FDA
Regulations for Process Validation; The pharmaceutical industry operates within a
stringent regulatory framework & adherence to FDA regulations is
non-negotiable.
 |
| Fig. 02: Navigating the Regulatory Landscape for Successful Process Validation in Pharma |
The FDA, as the regulatory authority overseeing
pharmaceuticals in the United States, plays a pivotal role in shaping the Pharmaceutical process validation Guideline.
Adhering to these regulations is non-negotiable for any pharmaceutical
manufacturer. The FDA regulations for process validation provide a framework
that emphasizes the importance of a well-defined and controlled manufacturing
process. Key elements include the validation
master plan, a comprehensive document that outlines the overall strategy
for the validation process.
Have an overview on Basics on regulatory aspects
Learn More…..
@ Pharmaceutical regulatory authorities All overthe world
—————————————————————————————————————————————
Step
3: Identifying Crucial Factors –
Critical Process Parameters At
the heart of successful process validation lies an in-depth understanding of critical process parameters (CPP).
Uncover the factors that can significantly impact the quality, safety, and
efficacy of pharmaceutical products, and learn how to manage and control them
effectively.
 |
| Fig. 03: Identifying Crucial Factors for Successful Process Validation in Pharma |
Critical
process parameters (CPPs) are the variables that have a direct
impact on the quality of the pharmaceutical product. Identifying and monitoring
these parameters are central to successful process validation. From temperature
and pressure to time and mixing speed, each parameter must be precisely
controlled to ensure the desired product quality. Understanding the critical process parameters sets the
stage for effective validation, enabling manufacturers to establish and
maintain control over the entire production process.
—————————————————————————————————————————————
Step
4: Crafting the Blueprint –
A validation master plan is
like the blueprint for ensuring everything in a process works smoothly. It covers what needs
validating, how to do it, and ensures all steps meet regulatory standards,
ensuring products are consistently top-notch.
 |
| Fig. 04: Crafting the Blueprint for Successful Process Validation in Pharma |
Validation
Master Plan (VMP), A standard Validation Master Plan Template
is required to serves as the roadmap for validation journey.
Explore the essential elements of a VMP, detailing how it provides a
comprehensive overview of the entire validation process, ensuring a systematic
and organized approach.
—————————————————————————————————————————————
Step
5: Unveiling the Lifecycle –
Process Validation Lifecycle Process validation
is not a one-time event; it’s a continuous lifecycle. Walk through each phase
of the process validation lifecycle, from design and qualification to continued
monitoring and improvement.
Understand how this cyclical approach ensures
ongoing compliance and product quality.
The process validation lifecycle is a continuous journey that begins
with the design phase and extends through the entire life of a product.
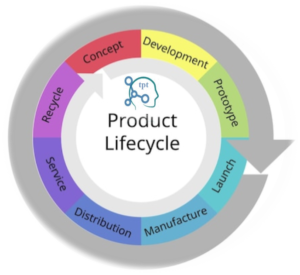 |
| Fig. 05: Unveiling the Lifecycle for Successful Process Validation in Pharma |
It comprises three stages: Stage 1 – Process
Design, Stage 2 – Process Qualification, and Stage 3 – Continued Process
Verification. Each stage plays a crucial role in ensuring the ongoing success
of process validation. This section will provide a detailed exploration of each
stage, highlighting the key activities and considerations at every step.
Have an overview on Basics on Lifecycle approach, Learn More…..
@ Whatis Product Lifecycle Management..? An Overview
@ Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery
—————————————————————————————————————————————
Step
6: Documenting the Journey –
Validation
Protocols and Reports Effective documentation is the backbone of
successful process validation. Learn the art of creating validation protocols and reports that not only satisfy regulatory
requirements but also serve as valuable tools for process improvement.
 |
| Fig. 06: Documenting the Journey for Successful Process Validation in Pharma |
Validation protocols are
detailed plans outlining how a process will be tested to ensure it meets
quality standards. Reports document the results of these tests, confirming that
the process consistently produces the desired outcomes. Together, they provide
a transparent record of the validation process, demonstrating compliance with
regulatory requirements and ensuring the reliability of product quality.
Learn More…..
Validationstudy: Why Three batch for validation study?
—————————————————————————————————————————————
Step
7: Ensuring Compliance –
Validation, Deviations and CAPA Even with
meticulous planning, deviations can occur. Explore the process of managing
validation deviations and implementing Corrective and Preventive Actions (CAPA)
to maintain compliance and enhance overall process reliability.
 |
| Fig. 07: Documenting the Journey for Successful Process Validation in Pharma |
Continuous process
verification involves ongoing monitoring and control to ensure that the process
remains in a state of control throughout commercial production. By adhering to
these validation principles, organizations can enhance product quality, reduce
the risk of defects, and ultimately achieve and maintain compliance with
regulatory requirements, fostering confidence in the reliability of their
processes and products.
—————————————————————————————————————————————
Step
8: Mastering Precision –
Mastering precision in the
context of process validation is a critical aspect that underscores the
meticulous control and optimization of manufacturing processes to ensure
consistent and accurate outcomes, which involves detailed mapping of process
parameters and their acceptable ranges.
Validation Equipment Qualification The equipment
used in pharmaceutical manufacturing plays a pivotal role in process success.
Dive into the details of equipment qualification, understanding how precise
calibration and validation ensure the accuracy and reliability of your
manufacturing processes.
 |
| Fig. 08: Mastering Precision for Successful Process Validation in Pharma |
By mastering precision in
process validation, industries can enhance product quality, reduce the
likelihood of defects, and adhere to regulatory requirements. This commitment
to precision not only ensures the reliability of manufacturing processes but
also fosters confidence in the reproducibility of high-quality products.
—————————————————————————————————————————————
Step
9: Integrating Statistical Wisdom –
Statistical
Process Control (SPC); SPC is a powerful tool for maintaining
consistent quality. Discover how statistical methods and data analysis
contribute to the ongoing monitoring and control of critical process parameters. Statistical
process control Software plays an important role in the execution of the
SPC like minitab, IQMS manufacturing ERP, WinSPC etc.
 |
| Fig. 09: Integrating Statistical Wisdom for Successful Process Validation in Pharma |
Integrating statistical
wisdom in process validation involves using statistical methods to gather
insights, analyze data, and enhance decision-making. It’s about harnessing the
power of statistics to ensure precision, reliability, and compliance in
manufacturing processes. By applying statistical tools, we gain a deeper
understanding of variability, optimize parameters, and maintain consistent
product quality. This approach fosters a data-driven culture that not only
meets regulatory standards but also enhances overall efficiency and confidence
in the validation process.
—————————————————————————————————————————————
Step
10: Achieving Continuous Improvement –
Continuous Process Verification The journey
doesn’t end with initial validation. Embrace the concept of continuous process
verification, a proactive approach that fosters ongoing improvement and ensures
the long-term success of pharmaceutical processes.
 |
| Fig. 10: Achieving Continuous Improvement for Successful Process Validation in Pharma |
Achieving continuous
improvement in process validation means consistently refining and enhancing
manufacturing processes. It involves a proactive approach to identifying and
implementing better methods, minimizing errors, and optimizing efficiency. This
ongoing commitment ensures that products meet high standards, comply with
regulations, and reflects a dedication to excellence in quality and reliability
—————————————————————————————————————————————
In Brief……..
By incorporating these 10 key steps into the pharmaceutical process validation
journey, it will not only meet regulatory requirements but also elevate the overall
quality and reliability of manufacturing processes. Here’s to achieving
excellence in every step of the way!
We Team
TPT Hope this comprehensive article certainly helped to enrich the
knowledge bucket of your mind..!! Keep reading………. J