What is Personalized Medicine ?
This is a revolutionary approach in healthcare that customizes medical treatment to the individual characteristics of each...

This is a revolutionary approach in healthcare that customizes medical treatment to the individual characteristics of each...
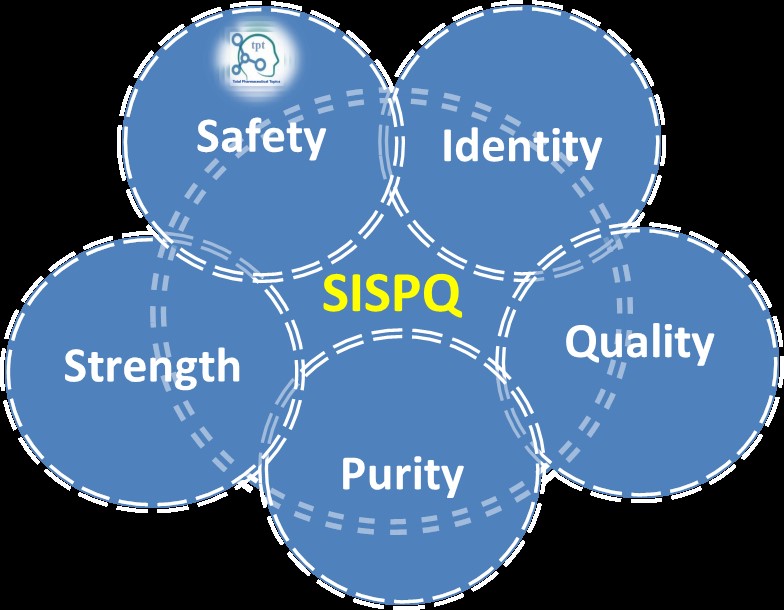
When we discuss the pharmaceutical manufacturing, SISPQ is very important topic of discussion. It adheres the basic...

These two terms are differ in scope and methodology, clinical trials and clinical research are essential components of...
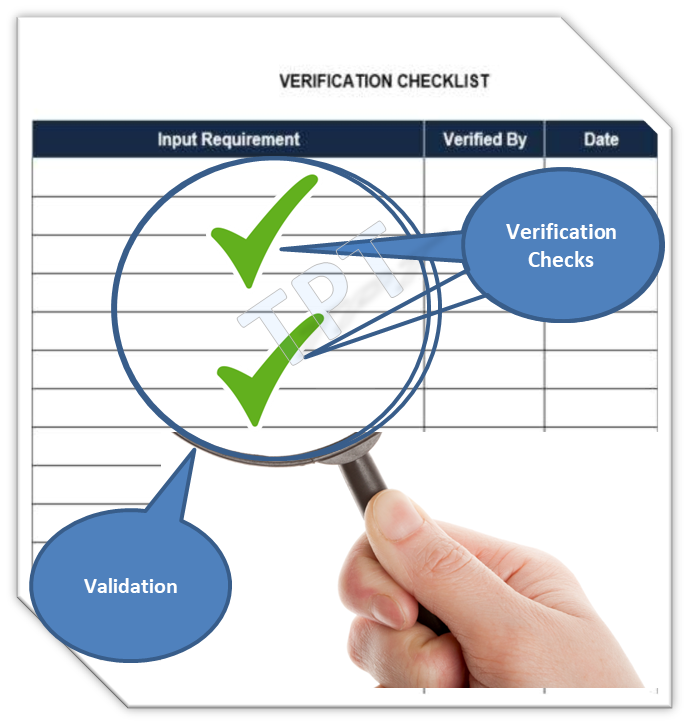
In the pharmaceutical industry, term verification and validation are being often used, this article is furnished just for...
What is Pharmaceutical Drug Substance / API (Active Pharmaceutical Ingredient) And Pharmaceutical Drug Products?This article...
Research and development is a process by which a organization acts to get new knowledge that it might use to create new...
Quality Policy; When we talk about the Quality management system (QMS) in pharmaceutical industry, Quality Policy is an...
