5 Common Issues Encountered During Process Validation and solutions
Process validation is a critical aspect of pharmaceutical manufacturing, ensuring that products meet their intended quality...
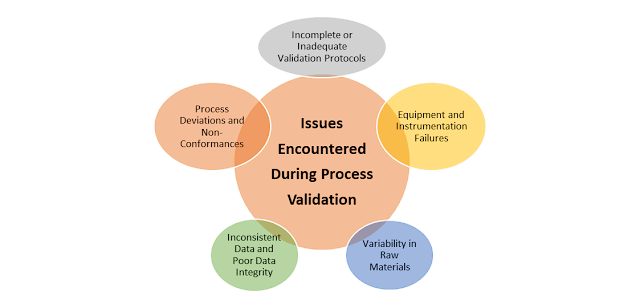
Process validation is a critical aspect of pharmaceutical manufacturing, ensuring that products meet their intended quality...

Pharmaceutical process validation is a critical aspect of drug substance manufacturing; that ensures the consistency and...
In the ever-evolving landscape of the pharmaceutical industry, ensuring the quality and reliability of processes...

Computer system plays a key role in many businesses from managing critical data to managing complex processes. The seamless...
In the previous article we got the introduction of Computer system validation, along with the approach objective and...
Computerized systems used in the various stages in pharmaceutical Industry. In the modern era Automation /...
In this article we will try to understand the fundamental concept of three batches for Validation study; or more correctly...
In previous article we understood what is pharmaceutical validation study and scope (phase)of validation. We...
Validation Study: Basic overview This Article is written here to have an overview on the validation Study, now...
This could be the basic question which is linked with the life of a product. In general term this is the life of a product...
