In the world of pharmaceuticals, the drug substance manufacturing process
is a critical component in bringing life-saving medicines to patients. This
complex process involves several steps, adherence to stringent rules, and
upholding the highest standards of quality.
This process is based on the basic
Good Manufacturing Practice (GMP) principles i.e. SISPQ; which means a drug
substance / drug product should be manufactured in such a way, that achieve Safety,
Identity, Strength, Purity and Quality.
In this article, we will go through with the various
phases from drug development through the ultimate delivery of the active pharmaceutical ingredient (API), taking a close look at the manufacturing process of drug
substances/ drug product.
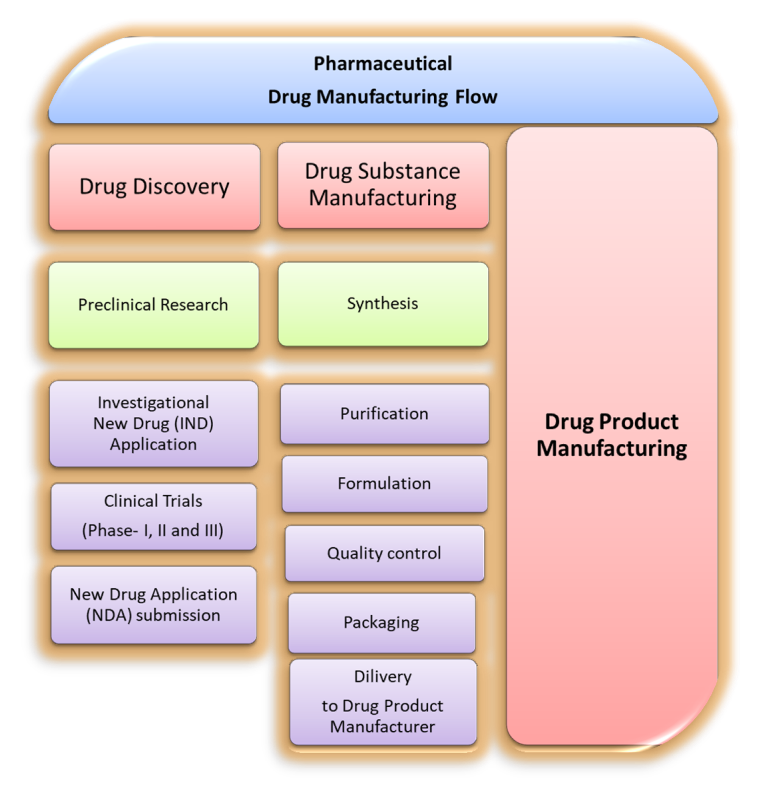
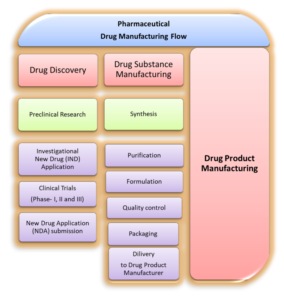 |
| Figure: 01: Pharmaceutical Drug Substance Manufacturing Flow: From Discovery to Delivery |
1. Drug Discovery
The process of producing drug substances starts
with drug discovery, in which pharmaceutical companies look into and find
prospective new chemicals entity (NCE) that might be used to treat particular medical
conditions.
To find the most promising options with the needed therapeutic
effects, scientists carefully examine thousands of chemicals. An identified
lead compound is put through extensive testing to determine its safety and
effectiveness.
 |
| Figure: 02: Drug Discovery |
2. Preclinical Research
As a result of drug discovery, once the lead compound
is identified, preclinical research starts. This stage involves a deep dive
study performed on cell-cultures and animal models to evaluate the compound’s
pharmacological characteristics, toxicity, and potential side effects.
The
objective of this stage is to collect the crucial data and vital information to
assist the “Investigational New Drug (IND)” submission process to
regulatory authorities.
 |
| Figure: 03: Preclinical Study |
3. Investigational New Drug (IND) Application
Once preclinical research establishes the safety
and efficacy of the drug candidate, the pharmaceutical company files an IND
application with the regulatory agencies.
This submission includes all relevant
preclinical data, proposed clinical trial protocols, and information on the drug
substance’s manufacturing process.
 |
| Figure: 04: Investigational New Drug (IND) Application |
4. Clinical Trials
Clinical trials are a set of carefully organized
experiments carried out on healthy people in order to evaluate the efficacy,
dose, and safety of the drug substance. These studies are conducted under
stringent restrictions & guidance.
This stage typically has four phases.
Phase I: focuses on establishing safety and dose,
Phase II: assesses efficacy
and further safety, and
Phase III: entails large-scale trials to validate
efficacy and track negative reactions.
Phase IV: Post-Market Surveillance
 |
| Figure: 05: Clinical Trial |
5. New Drug Application (NDA)
As the clinical trial study gets completed,
pharmaceutical companies put all the information collectively into a “New
Drug Application (NDA)”.
These information are obtained throughout the
aforementioned earlier stages of drug substance manufacturing. This thorough
dossier contains details on the creation of medicinal substances, including
information on preclinical research and clinical trial findings.
The regulatory
agencies are subsequently given the NDA to evaluate and approve.
 |
| Figure: 06: New Drug Application (NDA) |
6. Drug Substance Manufacturing
After receipt of regulatory approval, drug
substance manufacturing gets begins. The process involves multiple intricate
steps, including:
A Synthesis:
The drug’s API is synthesized in controlled laboratory environment /
conditions. The synthesis process must be consistent and reproducible, should
be documented properly to ensure consistent quality as per required acceptance
criteria.
B. Purification: The synthesized API is
purified to remove impurities and unwanted by-products. High-performance liquid
chromatography (HPLC) and other analytical techniques are employed to check and
monitor the purification step, to achieve the desired purity levels.
C. Formulation: Further the Active
Pharmaceutical ingredient (API) undergoes some additional processing steps to
get the final drug product, such as tableting, encapsulation, or suspension in
a liquid form.
D. Quality Control: Throughout the
manufacturing process, rigorous quality control checks are performed to ensure
the quality of API, which meets all required / desired quality specifications.
Any deviations are meticulously addressed to maintain product integrity.
E. Packaging: The final drug substance
(API) is carefully packaged and labeled, now at this stage it is ready for
distribution to various drug product manufacturers.
 |
| Figure: 07: Drug Substance Manufacturing |
7. Drug Product Manufacturing
Now, drug substance (API) supplied to drug
product manufacturer for formulating as final drug product. Formulation unit of
drug product manufacturer formulates the APIs into the final dosage forms, such
as tablets, capsules, or injectables.
The process of drug product manufacturing
process follows stringent quality standards & GMP (Good Manufacturing
Practices) guidelines.
 |
| Figure: 08: Drug Product Manufacturing |
8. Quality Assurance and Regulatory Compliance
Throughout the entire drug substance
manufacturing flow, quality assurance plays a vital role. Stringent quality
checks are conducted at every stage to comply with various regulatory requirements.
This ensures that the final product is safe, effective, and of
the highest quality.
 |
| Figure: 09: Quality Assurance and Regulatory Compliance |
The drug substance manufacturing flow is an
intricate and highly regulated process that plays a crucial role in bringing
life-saving medicines to patients worldwide.
From the early stages of drug
discovery to the final delivery of the API, each step demands precision,
compliance with strict regulations, and an unwavering commitment to quality.
Learn more……..
Pharmaceutical Drug substance (API) and Drug Product: Definition
Product Lifecycle management- An overview
Process Validation study- Types of validation and advantage
Pharmaceutical Purified water system : Fundamental Introduction
Computer system validation (CSV) in Pharmaceutical Industry !! Introduction