In
the world of pharmaceutical industry, Marketing Authorization term is
being used for formal approval process, by which various regulatory agency
grant permission for the commercial distribution and sale of a Pharmaceutical
Product. Marketing Authorization is an official document issued by the regulatory
agency for the purpose of marketing of a product after evaluation for Quality,
Safety and Efficacy.
What is Marketing Authorization in Pharmaceutical Industry?
The
Pharmaceutical Regulatory Agencies are responsible for the scientific
evaluation of centralised marketing authorisation applications (MAA). The
process for having marketing authorization for any pharmaceutical product is a
critical and rigorous step. Which goes with the concept of Product Lifecycle
management Process, i.e. from development stage to commercialization stage of
pharmaceutical products and further monitoring.
These
Marketing Authorization approval steps involves the submission of
wide-ranging data and information about the subjected product’s safety,
efficacy, and quality, gathered through preclinical and clinical trials, as
well as detailed information about the manufacturing processes.
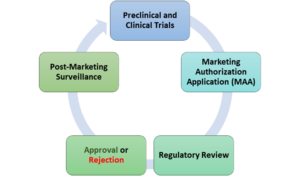
Key components of the Marketing Authorization process
Pre-clinical
and Clinical Trials:
Before
seeking marketing authorization, pharmaceutical companies conduct extensive preclinical
studies and clinical trials to gather data on the safety
and efficacy of the drug. This involves testing the drug in the laboratory and
on animals (preclinical) and then in humans (clinical). These trials form the
scientific backbone, providing essential insights into the safety, efficacy,
and overall quality of a product before it reaches the hands of healthcare
professionals and patients.
Marketing
Authorization Application (MAA):
Submitting
an MAA is a strategic and meticulous process, often involving collaboration
with regulatory experts. Once the necessary data has been collected, a
pharmaceutical company submits a Marketing Authorization Application to the
relevant regulatory agency. The application includes detailed information about
the drug’s composition, manufacturing process, proposed labelling, and the
results of preclinical and clinical studies. This comprehensive application is
a formal request submitted to regulatory authorities, seeking approval to
market and distribute a pharmaceutical or biotechnological product.
Regulatory
Review:
Once
Marketing Authorization Application (MAA) is submitted, the MAA undergoes a
rigorous review process by regulatory authorities, where experts assess the
provided data to ensure the product meets the necessary standards for safety,
efficacy, and quality. Regulatory agencies, such as the U.S. Food and Drug
Administration (FDA) in the United States, the European Medicines Agency (EMA)
in Europe, or other national regulatory bodies, review the MAA to ensure that
the drug is safe, effective, and of high quality. This review involves a
thorough assessment of the submitted data.
Approval
or Rejection:
Based
on the regulatory review, the regulatory agency makes a decision to either
approve or reject the marketing authorization. If approved, the pharmaceutical
company is granted permission to market and sell the drug for the specified
indications. If the regulatory authorities identify concerns related to safety,
efficacy, quality, or compliance with regulatory standards, they may reject the
MAA. Rejections can result from inadequacies in study design, data quality,
manufacturing processes, or failure to address regulatory queries adequately.
In
some cases, regulatory authorities may provide conditional approval, allowing
market entry under certain conditions. This is often based on a commitment from
the applicant to provide additional data post-approval. If an MAA is rejected,
applicants typically have the option to appeal the decision or resubmit the
application with additional data addressing the concerns raised during the
review process.
Post-Marketing
Surveillance:
Even
after marketing authorization is granted for pharmaceutical
product, ongoing monitoring of the drug’s
safety and efficacy continues through post-marketing surveillance. This helps
to identify and address any previously unrecognized issues that may arise once
the drug is in widespread use. It is an ongoing process designed to monitor the
safety, efficacy, and overall performance of the authorized product in
real-world conditions. Ensuring ongoing compliance with regulatory requirements
is a key aspect of Post-Marketing Surveillance.
If
we see the validity of Marketing authorisation as per various Pharmaceutical regulatory
agencies is valid for a period of 5 years and subjected for renewal after this
period. However, in the European Union, once the renewal has been done, the
marketing authorisation shall remain valid for an unlimited period, unless the
competent regulatory authority decides otherwise.
Learn More……
Drug Substance and Drug Product Manufacturing Flow: Discovery to Delivery
Drug Substance in Pharmaceuticals!! Unveiling Its Vital Role
The Evolution of Drug Substance Development
Clinical Trials: A Key to Pharmaceutical Development / Advancement
New Chemical Entities (NCEs) in Pharmaceutical Drug Development