Validation
Study: Basic overview
This
Article is written here to have an overview on the validation Study, now a
day’s validation became an integrated part of cGMP. As we all know that the
pharmaceutical industries are manufacturing the life saving drugs, hence the
key objective set by the pharmaceutical industry is to manufacture products of
required attributes and quality with consistency, at the lowest conceivable
cost.
Validation study is the first requirement of any regulatory authority to
give approval of commercialization / Market authorization. Various regulatory
authorities defined the guidance on the Validation study and its approach for execution;
here in this article we are going to have an overview on complete validation
study.
Inside Story
- What
is validation study? - History
of Validation. - Scope
(Phases) of validation.
What is Validation study?
term VALIDATION is self explanatory i.e. “This is a process to prove VALID in
terms of consistency of a particular operation/process”. This is an assessment
process for validity / effectiveness of series of operations. Documented
evidence is being generated throughout the study program, with the reference of
guidance documents of various Pharmaceutical regulatory authorities all over the world.
principal goal of Pharmaceutical Manufacturer is to manufacture product with
the desired /requisite quality. Operational / Mechanical consistency plays a
major role to achieve the prime goal of pharmaceutical manufacturing. This
Operational / Mechanical consistency need to be proved. Validation study also
involves some extra cost to the manufacturing, as this is an extensive study
program to prove the consistency / effectiveness of an operation/process.
prove control on quality of manufactured product, it is very essential to
perform validation study. A process that is well understood and in the state of
confidence, control of product quality of the product manufactured cannot be assured
without validation program.
History
of validation study
Concept
of validation is primarily derived from US FDA (Food and drug association)
regulations describing the current good manufacturing practice (cGMP) for finished
pharmaceuticals (21 CFR parts 210 and 211).
USFDA was the pioneer to advocating
the concept of validation. In the mid of 1970’s this concept was proposed by
two FDA officials names Ted Byers and Bud Loftus. This concept was introduced
looking at the several problems in the sterility of large volume parental
Market. First draft guidance on General principles of Process validation was come
in May 1987.
As we
defined, in the above section the term validation is the process to prove operational
and mechanical consistency, scope of validation study program is very vast, it
covers every aspect of the pharmaceutical industry from its establishment to
commercialization of product. An organized overview on validation study scope
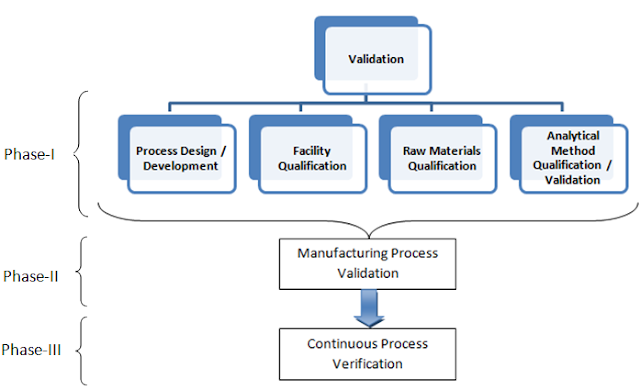
defined here, which involves three phases of validation;
- Phase-I: Pre-validation
/ Qualification Phase (Prerequisites of Process validation) - Phase-II:
Validation Execution Phase (Process Validation) - Phase-III:
Validation Maintenance Phase (Continuous process verification)
- Process
Design / Development: This Study is comprise of various Process Understanding
Studies which includes; Process control study, Hold time study, In-process
control study, Filtration study, Drying
Study, Shipping validation, Process Data Trending etc.
- Facility
Qualification: This study is comprised of the study on various parts of a manufacturing
facility which allows manufacturing of product within; likewise Utility
Qualification, Equipment Qualification and calibration study program, Equipment
Cleaning Validation, Operator Qualification etc.
- Raw
Materials Qualification: Raw material Qualification study comprises of Raw
material Analysis, Vendor/supplier Qualification and Transportation validation
study etc.
- Analytical
Method Qualification / Validation: Analytical Method Qualification study involves
the qualification study for the analytical methods Residuals Assay
Qualification, In-process Method Validation, Release Method Validation etc.
Phase-II:
Validation Execution Phase (Process Validation)
In
the first phase of validation i.e. Pre-validation / Qualification Phase we have
understood the things need to be established prior to validate a manufacturing
process of a drug substance (API) / Drug Product. This study is designed to validate or
authenticate that an established sequence of process, established acceptance
criteria and critical / non critical process parameters are suitable and
adequate.
This study is a type of declaration that particular process can
produce with the consistency operation / procedural and quality point of view over
the period of time even under the worst conditions.
Phase-III:
Validation Maintenance Phase (Continuous process verification)
This
is the maintenance phase of manufacturing process of drug substance (API) /
Drug Product.
As the name suggest it requires a frequent review of all process/product
related documents which involves Manufacturing process, Quality management
system (QMS) documents, analytical documents etc. Intention for the review of
all this documents is to ensure that it have been no changes have been made /
occurred in the process after execution of Phase-II i.e. process validation
study, No Failure, deviations and modification took place without any
investigation or acknowledgement.
Team comprising of representatives of major departments ensures that there is
no impact on the existing validation study performed in the Phase-II. No such deviation/failure
occurred that impacted the validation study and ought to recommend performing
re-qualification re-validation study.
This maintenance study could be an alert system
design and validation of the system that shows high degree of assurance that
all the lots and batches manufacture will achieve their particular established
specifications.
I
hope this article on basic introductory knowledge on validation study in pharmaceutical industry, helps you to boost up your knowledge stock of your mind.
Other useful
articles may informative…..
- Product
Lifecycle Management: An Overview - Pharmaceutical
regulatory authorities All over the world - Pharmaceutical
Quality Management System: An overview - What
is Quality Management system for pharmaceutical industry? - Elements
of Quality management system: An overview - Pharmaceutical
Drug substance (API) and Drug Product: Definition - Research
and Development: Definition


Very informative and nicely presented.
Thank you sir….!!