What is Personalized Medicine ?
This is a revolutionary approach in healthcare that customizes medical treatment to the individual characteristics of each...

This is a revolutionary approach in healthcare that customizes medical treatment to the individual characteristics of each...
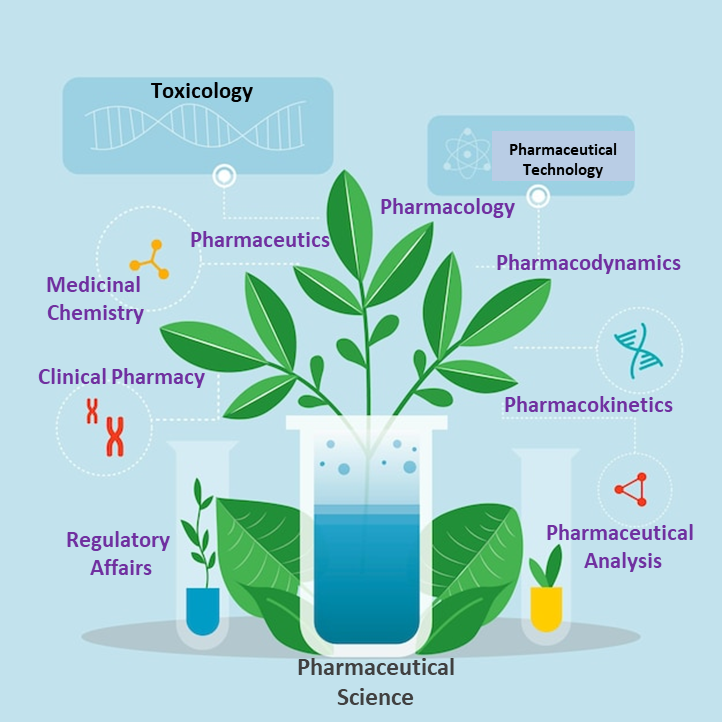
Pharmaceutical science covers a diverse field focused on the discovery, development, and evaluation of drugs intended for...
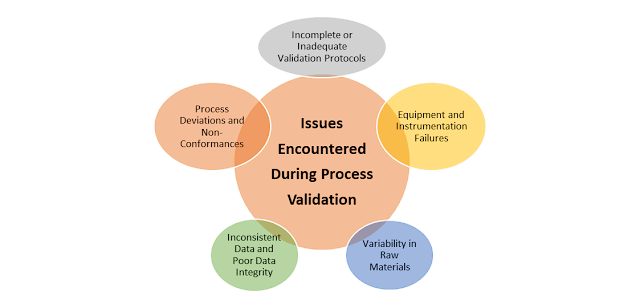
Process validation is a critical aspect of pharmaceutical manufacturing, ensuring that products meet their intended quality...
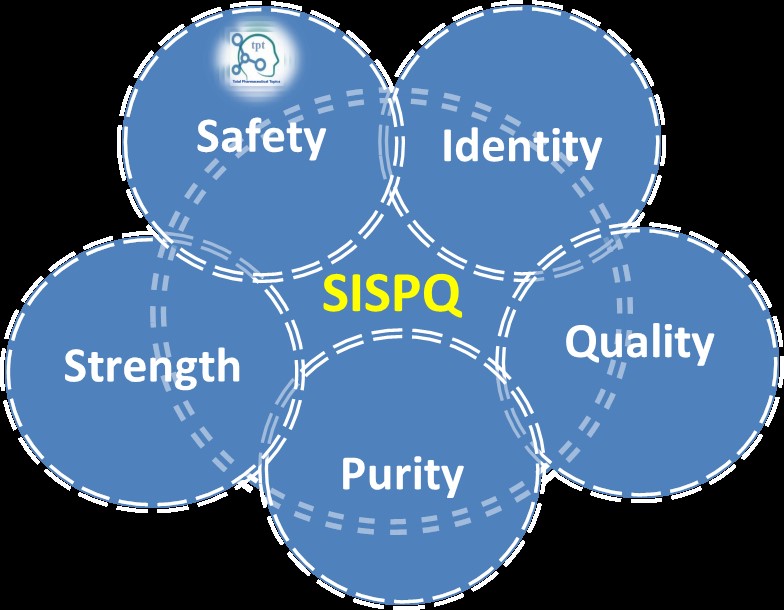
When we discuss the pharmaceutical manufacturing, SISPQ is very important topic of discussion. It adheres the basic...
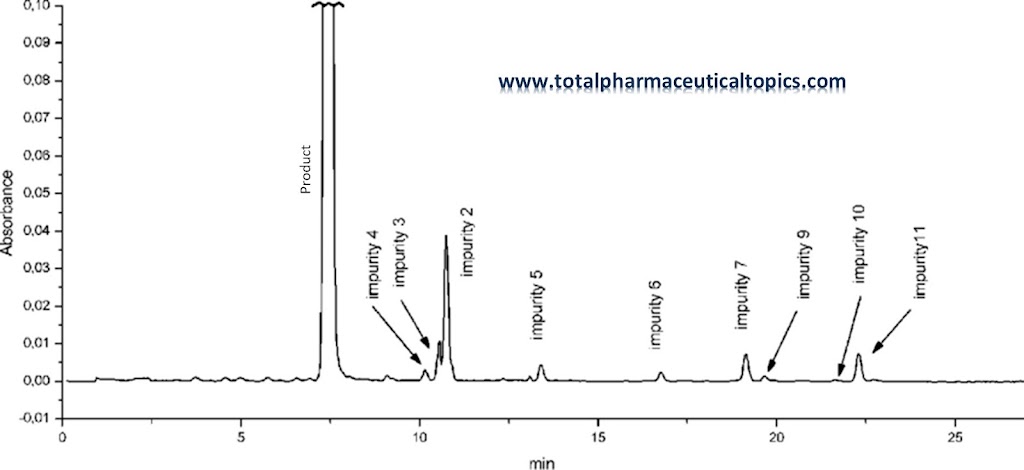
In General terms Impurity is referred as something, which is not pure. Some substance / component that may affect the...

Regulatory Affairs is a profession, known as a bridge / a channel of communication, between the Regulatory Agency and the...

The concept of Green Chemistry accepted by the scientific community, the technical Green Chemistry evolution is yet to...
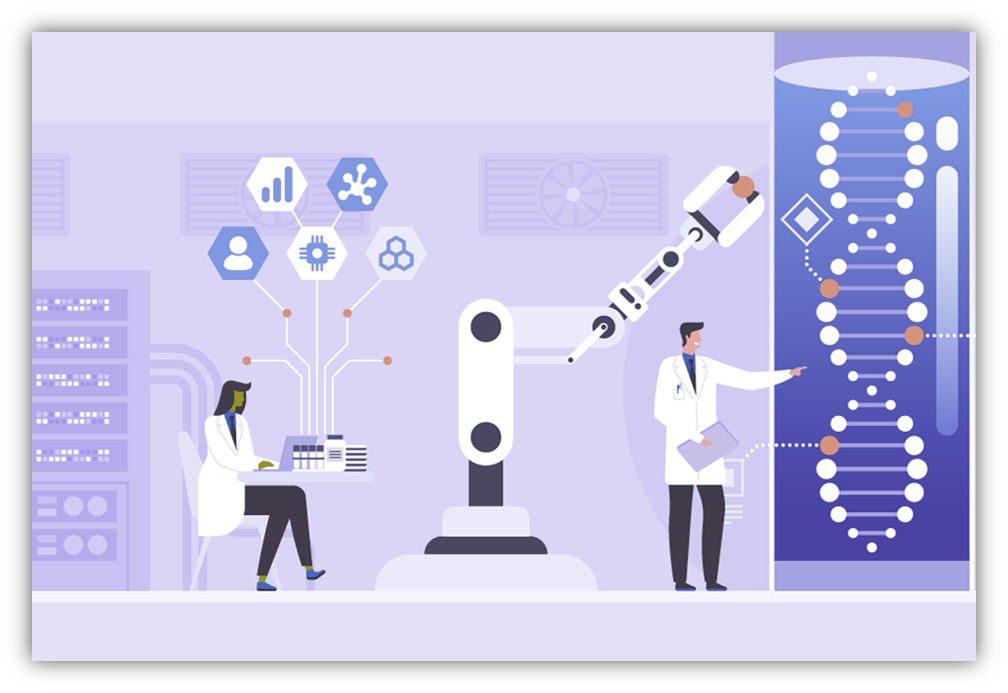
Artificial Intelligence and Automation in Healthcare, these are key step for Revolutionizing...

These two terms are differ in scope and methodology, clinical trials and clinical research are essential components of...

In the world of pharmaceutical industry, Marketing Authorization term is being used for formal approval process, by which...
