Process validation is a critical aspect of
pharmaceutical manufacturing, ensuring that products meet their intended
quality standards consistently.
However, the path to successful process
validation is often troubled with challenges. In this article, we will explore
common issues encountered during process validation and provide practical
solutions to overcome them.
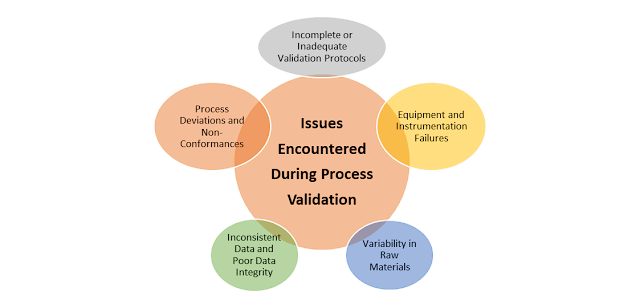
1. Incomplete or Inadequate Validation Protocols.
2. Inconsistent Data and Poor Data Integrity
3. Equipment and Instrumentation Failures
4. Variability in Raw Materials
5. Process Deviations and Non-Conformances
 |
| Issues Encountered During Process Validation and solutions |
Incomplete or Inadequate Validation Protocol
Lack of comprehensive documentation can hinder
the validation process. Incomplete or poorly organized records make it
difficult to demonstrate compliance with regulatory requirements.
Among this, one of the most significant
challenges in process validation is developing robust validation protocols.
Common issues include a lack of clear objectives, poorly defined acceptance
criteria, and insufficient procedural details.
Have a Validation Master Plan and specific
procedure to guide you as to the who, what, when, why of validation at your
facility, and how those validations are conducted.
SOLUTION:
🔑 Develop comprehensive validation protocols with
clearly defined objectives, detailed procedures, and specific acceptance
criteria.
🔑 Ensure protocols are reviewed and approved by
qualified personnel.
🔑 Use standardized templates for protocols.
Inconsistent Data and Poor Data Integrity:
Data integrity is utmost requirement in process
validation execution. Inaccurate data recording, inadequate data verification,
and compliance issues with electronic data management systems can compromise
validation efforts.
High-integrity
data in process validation studies is crucial for ensuring regulatory
compliance, product quality, operational efficiency, and overall company
reputation.
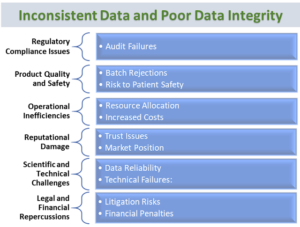 |
| Inconsistent Data and Poor Data Integrity |
SOLUTION:
🔑 Implement robust data integrity practices, such
as the ALCOA+ principles (Attributable, Legible, Contemporaneous, Original,
Accurate, Complete, Consistent, Enduring, and Available).
🔑 Utilize validated electronic data management
systems and conduct regular audits to ensure compliance.
Equipment and Instrumentation Failure:
Equipment failures are common during validation,
often due to poor maintenance, lack of calibration, and operational issues
during qualification phases. This state can
significantly obstruct the accurate assessment of manufacturing processes.
Additionally,
equipment failure introduce variability into the process, hindering the
evaluation of its true performance and consistency. Addressing these failures
drains valuable resources and poses risks to product quality if left
unattended.
SOLUTION:
🔑 Regularly calibrate and maintain equipment.
that equipment is functioning correctly before validation activities begin.
🔑 Review periodically the validation, calibration
and maintenance activity, via setting up the schedule.
During Validation protocol preparation and
review, verify the calibration and validation status, should be within the
acceptance criteria of respective schedule.
Variability in Raw Materials:
significant variability in the validation process. Supplier-related issues
often intensify this problem.
Variability
in raw materials can introduce additional complexity into the manufacturing
process, making it harder to maintain consistent control over process
parameters. This can lead to variations in critical quality attributes (CQAs)
and critical process parameters (CPPs).
High
variability can increase the likelihood of batch failures due to deviations
from quality standards, resulting in significant financial losses and potential
supply chain disruptions.
SOLUTION:
🔑 Pharmaceutical
companies should implement robust raw material control strategies, including; stringent
supplier qualification, continuous monitoring, control strategy, and
comprehensive validation studies to mitigate the risks associated with raw
material variability.
🔑 Conduct thorough supplier qualification and
audits. Establish quality agreements with suppliers to ensure consistent raw
material quality. Perform rigorous raw material testing and qualification
before use.
Process deviations and non-Conformances:
Critical concepts like Process Deviations and
Non-Conformances must
be carefully managed.
These
deviations can be intentional or unintentional and can occur at any stage of
the manufacturing process and Non-conformances are usually identified through
inspections, testing, audits, or quality control checks.
Deviations from established procedures can occur
during validation, These
deviations and non-conformances can arise from various sources, including
equipment malfunctions, human error, or unforeseen environmental factors.
SOLUTION:
🔑 Establish a robust deviation reporting and
management system.
deviation.
🔑 Thorough
documentation of deviations and non-conformances, along with the corresponding
corrective actions, is essential for maintaining compliance with regulatory
standards and ensuring the integrity of the validation process.
IN SUMMARY…………….
Addressing these common issues with effective
solutions can significantly enhance the success and reliability of process
validation efforts in the pharmaceutical industry.
By implementing robust protocols, ensuring data
integrity, maintaining equipment, managing risks, and staying compliant with
regulatory guidelines, companies can overcome these challenges and achieve
consistent product quality.
For more insights and detailed guidelines on
process validation, stay tuned to our blog and follow our updates on
pharmaceutical manufacturing best practices.
LEARN MORE………..
What is Validation study- Basic overview
Process Validation study- Types of validation and advantage
Validation study: Why Three batch for validation study?
What is Difference between Verification and Validation in Pharmaceutical Industry??
5 Common Challenges in Pharma Drug substance Process Validation