What is Regulatory Dossier in Pharmaceutical Industry?
Regulatory Affairs is a profession, known as a bridge / a channel of communication, between the Regulatory Agency and the...

Regulatory Affairs is a profession, known as a bridge / a channel of communication, between the Regulatory Agency and the...
Artificial Intelligence and Automation in Healthcare, these are key step for Revolutionizing...

In the world of pharmaceutical industry, Marketing Authorization term is being used for formal approval process, by which...

When it comes to the world of pharmaceuticals, you may have heard the term "New Chemical Entity" / NCE under Drug...
Introduction to Clinical Trial? When we talk about the pharmaceutical development, Clinical trial is a...
The development of drug substances has evolved significantly over the years. In the past, drug substances /API ...
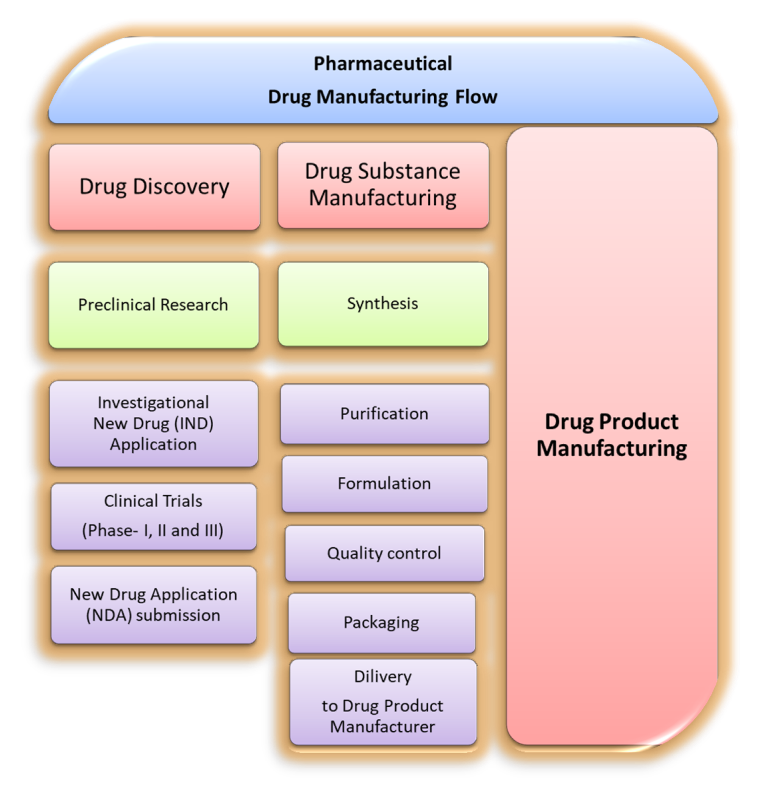
In the world of pharmaceuticals, the drug substance manufacturing process is a critical component in bringing life-saving...
