New Chemical Entities (NCEs) in Pharmaceutical Drug Development
When it comes to the world of pharmaceuticals, you may have heard the term "New Chemical Entity" / NCE under Drug...

When it comes to the world of pharmaceuticals, you may have heard the term "New Chemical Entity" / NCE under Drug...

Pharmaceutical process validation is a critical aspect of drug substance manufacturing; that ensures the consistency and...
In the ever-evolving landscape of the pharmaceutical industry, ensuring the quality and reliability of processes...
Introduction to Clinical Trial? When we talk about the pharmaceutical development, Clinical trial is a...

A strong and effective quality management system (QMS) is perhaps more important than ever in the fast changing...
The development of drug substances has evolved significantly over the years. In the past, drug substances /API ...

In the intricate world of pharmaceuticals, every pill, capsule, or vial of medicine begins its journey as a carefully...

Computer system plays a key role in many businesses from managing critical data to managing complex processes. The seamless...
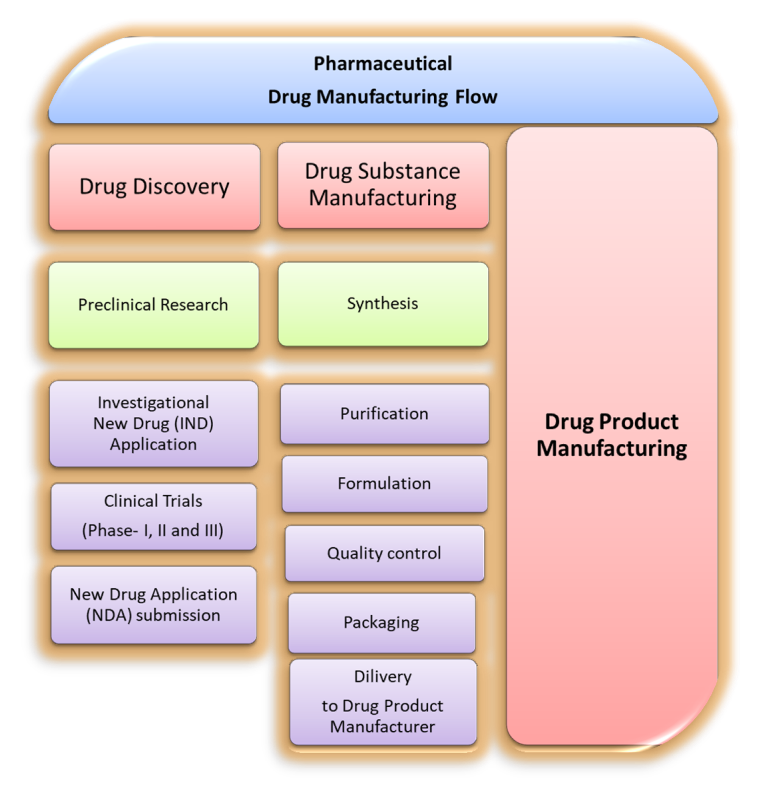
In the world of pharmaceuticals, the drug substance manufacturing process is a critical component in bringing life-saving...
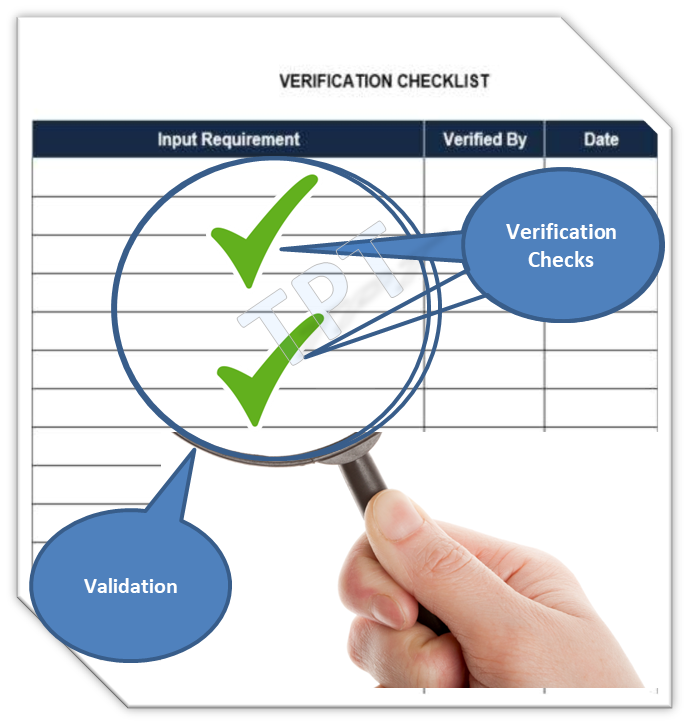
In the pharmaceutical industry, term verification and validation are being often used, this article is furnished just for...
